Using
Cysview®
Professional Guidelines
Guidelines recommend using Blue Light Cystoscopy (BLC®) with Cysview1,2
The American Urological Association (AUA)/Society of Urologic Oncology (SUO) state in their joint 2016 guideline (amended 2020):1
“In a patient with NMIBC, a clinician should offer blue light cystoscopy at the time of TURBT, if available, to increase detection and decrease recurrence. (Moderate Recommendation; Evidence Strength: Grade B).”1
AUA/SUO Guideline1
Diagnosis
- “At the time of resection of suspected bladder cancer, a clinician should perform a thorough cystoscopic examination of a patient’s entire urethra and bladder that evaluates and documents tumor size, location, configuration, number, and mucosal abnormalities. (Clinical Principle).”
- “At initial diagnosis of a patient with bladder cancer, a clinician should perform complete visual resection of the bladder tumor(s), when technically feasible. (Clinical Principle).”
- “A clinician should perform upper urinary tract imaging as a component of the initial evaluation of a patient with bladder cancer. (Clinical Principle).”
- “In a patient with a history of NMIBC with normal cystoscopy and positive cytology, a clinician should consider prostatic urethral biopsies and upper tract imaging, as well as enhanced cystoscopic techniques (blue light cystoscopy, when available), ureteroscopy, or random bladder biopsies. (Expert Opinion).”
Risk stratification
- “At the time of each occurrence/recurrence, a clinician should assign a clinical stage and classify a patient accordingly as “low-,” “intermediate-,” or “high-risk.” (Moderate Recommendation; Evidence Strength: Grade C).”
National Comprehensive Cancer Network Clinical Practice
Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer
(Updated 2023)2
The NCCN Guidelines state:
- Enhanced cystoscopy may be helpful in identifying lesions not visible using white light cystoscopy.
- Consider enhanced cystoscopy (if available) for initial evaluation or when positive urine cytology.
Required Equipment
Cysview® is FDA-approved for use with the KARL STORZ D -
Light C Photodynamic Diagnostic (PDD) system3
Blue Light Cystoscopy is now available on a per-case
basis from ForTec Medical. Contact us to learn more.
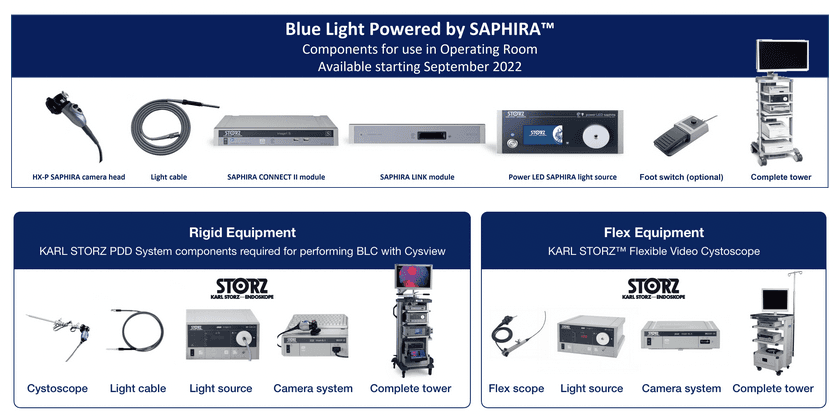
Blue Light Powered by SAPHIRA™
Launched in September 2022, the Blue Light Powered by SAPHIRA™ PDD system offers state-of-the-art
image quality and practical features to make the technology more user friendly.
Equipment features and benefits:
- High-definition image enables razor-sharp image quality for greater precision in the blue-light mode.
- LED light source provides more consistent light quality that won’t degrade over time.
- Fiber-optic cable gives you convenient autoclaving options.
- CHROMA setting allows more visualization of vascularity.
- Ergonomic camera head offers blue-light intensity control.
Procedure benefits:
- Simplified set-up process - there’s no more start-up sequence.
- More reliable parts - more durable light source and cable, which means fewer surprises during a case.
- Even better visibility - when you can see better, you can have more confidence in the quality of the TURBT.
Equipment components
To perform Blue Light Cystoscopy (BLC®) with Cysview as an adjunct to White Light Cystoscopy, you will need
the KARL STORZ system components listed below.
Support resources
Staff involved in the process also receive appropriate training.
While there is a learning curve associated with gaining expertise in this technology, there are help and
resources always available. Please see below:

The Operating Room Guide
The Operating Room Guide provides important information on the set-up and use of the non-SAPHIRA™ equipment, including troubleshooting steps.
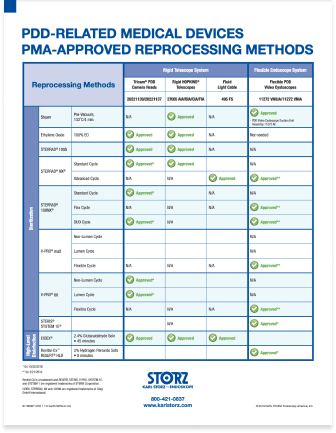
The PDD Reprocessing Guide
The PDD Reprocessing Guide is a quick and easy reference tool for understanding which reprocessing methods are premarket-approved for which PDD-related medical devices.
Reconstitution and instillation
This video walks you through the steps of Cysview reconstitution and instillation.
See our additional resources below:
Hover over materials below to download or order printed copies.
The Reconstitution and Instillation Guide provides step-by-step instructions for reconstituting and instilling Cysview
The Reconstitution Poster can hang on your wall to provide easy reference to the step-by-step instructions for reconstituting Cysview
For questions about or assistance with the Cysview reconstitution and instillation process, speak
with your Cysview Account Manager or contact us.
Nurse Support Team for Blue Light Cystoscopy
(BLC®) with Cysview®
Photocure: The Bladder Cancer Company has a Nurse Support Team available to respond one-on-one to your individual needs.
You can connect directly with our nurses for help with all your Cysview questions.
The goal of the Cysview Nurse Support Team is to help ensure that you have everything you need to make BLC with Cysview procedures
run smoothly at your facility. They are available to answer your questions and patient questions.
-
Tara Cumming
MSN, AGACNP-BC
Texas Urology Group -
Sarah Park
RN, BSN
Atlantic Urology Clinics -
Niki Osimo
LVN
USC Dept. of Urology

Tara Cumming
Tara Cumming, MSN, AGACNP-BC is a Nurse Practitioner at Texas Urology Group, San Antonio, TX. She specializes in urologic oncology. Tara works with Cysview patients in the clinic providing education in the preoperative stage and assisting with postoperative patient management. Her experience with BLC with Cysview began in 2017 at Vanderbilt University Medical Center, Nashville, TN, in the Department of Urologic Surgery.
She attended the Catholic University of America in Washington, DC, where she received her Bachelor of Science in Nursing. Tara then went on to practice as a Registered Nurse in med/surg, intensive care, and hematology. She earned her Master of Science in Nursing with a subspecialty in Adult Acute Care from Thomas Jefferson University in Philadelphia, PA. Tara is a board-certified, adult-gerontology, acute-care nurse practitioner licensed in Tennessee. She has worked primarily in urology with both general urologic conditions and urologic oncology.

Sarah Park
Sarah Park, RN, BSN is the Director of Nursing for Atlantis Urology Clinics, Myrtle Beach, SC. She assists with pre-, intra-, and post-op care of Cysview patients in the office. Her responsibilities include scheduling, education, instillation, assisting with Blue Light Cystoscopy procedures, documentation and billing, processing of instruments, and supply ordering. Sarah oversees the nursing staff and works with six urologists and four mid-level HCPs across three offices. She also works with BCG and other chemotherapy bladder installations – often on patients who had a lesion identified using Cysview.
Sarah earned her Bachelor of Science in health education and promotion from East Carolina University, Greenville, NC. After that, she earned her associate’s degree in nursing from Horry Georgetown Technical College in Myrtle Beach. More recently, Sarah received her bachelor’s in nursing from Western Governors University online.

Niki Osimo
Niki Osimo, LVN works as a urology nurse in the position of clinical manager at USC Department of Urology, Los Angeles, CA. There, she has been assisting leading bladder cancer expert Dr. Sia Daneshmand in the use of Blue Light Cystoscopy (BLC®) with Cysview since 2018.
Upon graduation from American Career College in Los Angeles in 2010, Niki began her nursing career at UCLA in the Frank Clark Department of Urology, also in Los Angeles.
In her current role, Niki gets to be at the patient’s bedside during the BLC procedure. This opportunity to provide comfort and reassurance to patients in time of need is what motivated her to become a nurse in the first place. Observing the procedure first-hand has shown her how beneficial it can be for patients’ overall well-being in addition to their bladder cancer management. USC averages approximately 400 Blue Light Cystoscopies in clinic per year
When to use Cysview®
Recommended use cases4-6
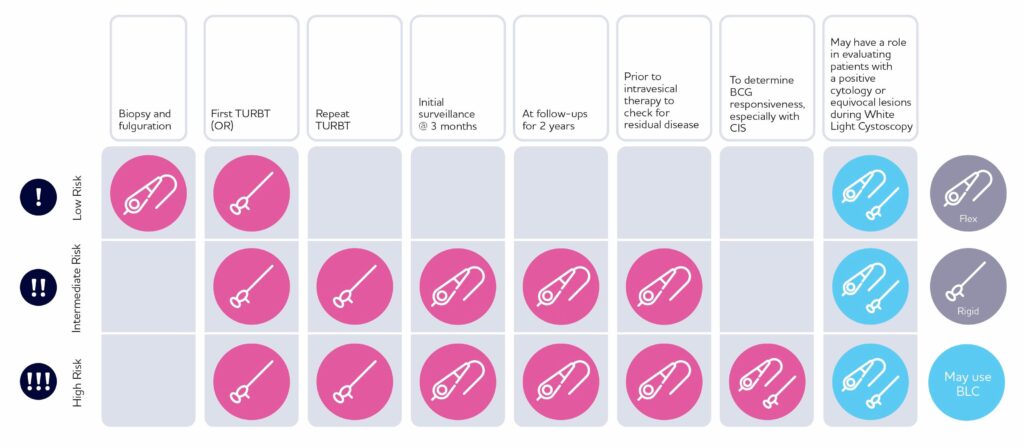
Use Blue Light Cystoscopy with Cysview in patients with non-muscle invasive bladder cancer.
Use in first TURBT and the management of intermediate and high-risk patients.
Example use cases from bladder cancer experts consensus publications
- At initial TURBT on suspicion of NMIBC.
- For repeat TURBT.
- Being checked to assess response to BCG therapy 6 weeks after completion.
- Undergoing surveillance.
- Prior to intravesical therapy when residual disease is suspected (the patient has not previously had BLC with Cysview).
- With positive cytology and negative White Light Cystoscopy (WLC).
Guideline and expert consensus recommendations
At initial TURBT on suspicion of NMIBC
AUA/SUO Guideline:
In a patient with NMIBC, a clinician should offer BLC with Cysview, if available, to increase detection and decrease recurrence. (Moderate Recommendation; Evidence Strength: Grade B)1
Pivotal clinical study:
From a prospective, comparative, within-patient controlled, multicenter phase III study in the detection of Ta/T1 tumors in patients who had previously undergone a cystoscopy and had suspicion of or confirmed NMIBC:
Out of 286 patients with at least one Ta or T1 tumor, 16% had additional Ta or T1 tumors only detected with BLC with Cysview (p=0.001).7
2018 Consensus Panel of NMIBC experts:
Strong recommendation to use BLC with Cysview.6
For repeat TURBT
2018 Consensus Panel of NMIBC experts:
BLC with Cysview with either flexible or rigid scope is strongly recommended in intermediate and high-risk patients.6
Following treatment with BCG
2014 Consensus Panel of NMIBC experts:
The decision to use BLC with Cysview in this instance should be made on a patient-by-patient basis by taking into account the benefit from accurately diagnosing more clinically significant disease cases versus the risk of false positives. The incidence of false positives decreases as time from BCG therapy increases.5
2018 Consensus Panel of NMIBC experts:
This scenario is an important endpoint for flexible and rigid BLC with Cysview in high-risk patients.6
In surveillance
Pivotal clinical study:
From a prospective, comparative, open-label, within-patient controlled, multicenter phase III study in the detection of bladder cancer during surveillance (n=63):
At surveillance, 21% of recurrent patients were only found with BLC with Cysview (p<0.0001).8
Cysview® may not detect all bladder tumors and is not a replacement for random biopsies.
2018 Consensus Panel of NMIBC experts:6
For intermediate-risk patients
- At 3-month surveillance with flexible scope
- At HCP-determined frequency during surveillance for 2 years
For high-risk patients
- At 3-month surveillance with flexible scope
- At 6-month follow-up surveillance with flexible scope
- At every other follow-up for 2 years
Prior to intravesical therapy when residual disease is suspected (the patient has not previously had BLC with Cysview)
2018 Consensus Panel of NMIBC experts:
A majority of the panel (13/17) thought that BLC with Cysview would be of benefit before initiating intravesical therapy in patients at intermediate risk or high risk of recurrence, based on the patient’s not having undergone a previous TURBT using BLC with Cysview.6
When positive cytology and negative WLC
AUA/SUO Guideline:
In patients with a positive cytology and negative WLC, a clinician should consider prostatic urethral biopsies and upper tract imaging, as well as enhanced cystoscopic techniques (BLC, when available), ureteroscopy, or random biopsies. (Expert Opinion).1
2018 Consensus Panel of NMIBC experts:
Flexible BLC with Cysview may be used in low, intermediate and high-risk patients. The panel agreed with the AUA/SUO NMIBC Guideline on the need for biopsy, but also considered that flexible BLC could be helpful in determining which operative procedure might be necessary.6
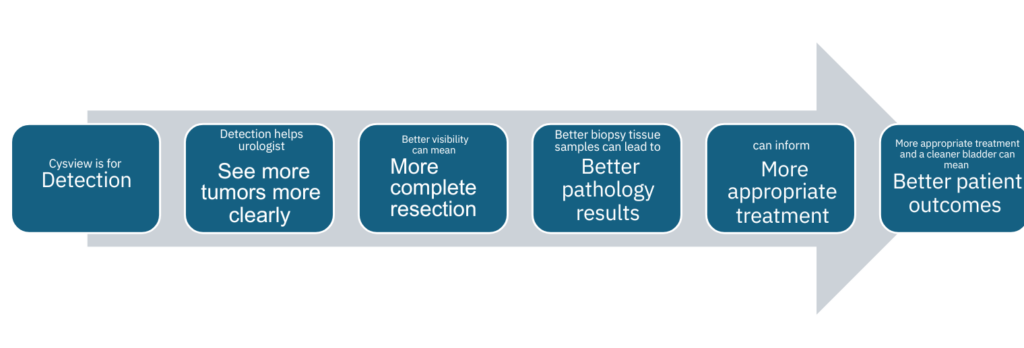
More-complete TURBTs can lead to improved patient outcomes
Optimal visibility during a TURBT can help urology surgeons perform a more complete resection. More
tissue removal provides more samples for the pathologist to examine. More data can lead to more accurate
diagnosis. The sooner a patient is correctly diagnosed, the sooner the urologist can administer the most
appropriate treatment. As a result, patient outcomes may improve and improve sooner.
Expert tips and experience with Cysview®
A quick overview of tips and tricks for Blue Light
Cystoscopy (BLC®) with Cysview®
Observation
Cause
Solution
Visual
Weak fluorescence
- Equipment failure
- Blood in the bladder
- Inadequate time
- Air bubbles
- Concealed tumors
- Make sure equipment is working and connected properly.
- If blood is present, remove resectoscope and flush using a bladder syringe attached to the trocar.
- Ensure that Cysview was instilled 1 hour prior to cystoscopy.
- Remove any air bubbles.
- Look behind any folds.
No fluorescence
- The equipment has not been set up correctly
- Blue light is not activated
- No malignant lesions present
- Ensure you are using correct equipment; look for blue or violet marker.
- Make sure Cysview has been instilled, and check for fluorescence in bladder neck.
- Inspect light source; original light bulb?
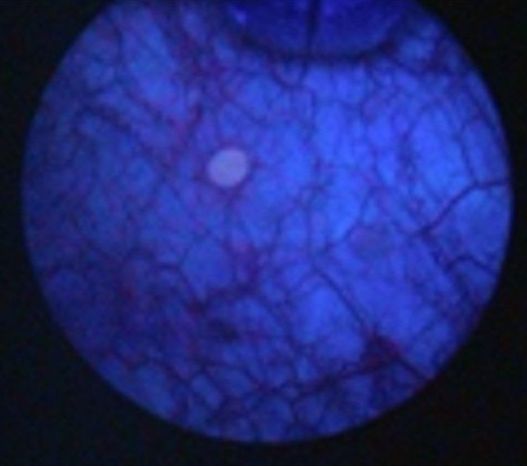
Green hue
- Urine in the bladder
- Always drain the bladder at start of the procedure.
- Remove resectoscope, set trocar valve to exit position, and allow urine to drain passively.
Entire bladder appears
red under white and blue
light
red under white and blue
light
- Recent BCG treatment or inflammation
- If clinically feasible, avoid BLC with Cysview until 6 weeks after last BCG treatment and in patients with bladder infection.
- Either continue procedure without the benefit of blue light diagnosis or reschedule.
Photobleaching
- Prolonged use of blue light
- Blue light too close to lesion during procedur
- Alternate between white light and blue light. If the tumour is visible in white light, use BLC with Cysview for control after resection.
- Do not use blue light close to the tumour for extended time periods. If photo bleaching appears, shut off the blue light and work elsewhere.
- Mark small lesions early during procedure.
Uncertainty around a
large pink/red area
large pink/red area
- Direct scope 90° toward lesion. Fill bladder slightly. Stretch area with loop and see if it disappears.
Questions:
- Has a cytology been taken? Were bacterial cultures taken? Has patient used catheter for longer periods?
- Is carcinoma in situ (CIS) suspected? Cold cup biopsy and no fulguration
- Did the patient have a positive urine cytology? Treat as CIS.
Trouble finding orifice or
suspicion that orifice
might have been resected
suspicion that orifice
might have been resected
- Switch to blue light and wait in suspected area; you might see a green cloud emerging because urine appears fluorescent green under blue light.
Weak
fluorescence
fluorescence
- Equipment failure
- Blood in the bladder
- Inadequate time
- Air bubbles
- Concealed tumors
- Make sure equipment is working and connected properly.
- If blood is present, remove resectoscope and flush using a bladder syringe attached to the trocar.
- Ensure that Cysview was instilled 1 hour prior to cystoscopy.
- Remove any air bubbles.
- Look behind any folds.
No
fluorescence
fluorescence
Green hue
Entire bladder
appears red
under white
and blue light
appears red
under white
and blue light
Photobleaching
- Prolonged use of blue light
- Blue light too close to lesion during procedur
- Alternate between white light and blue light. If the tumour is visible in white light, use BLC with Cysview for control after resection.
- Do not use blue light close to the tumour for extended time periods. If photo bleaching appears, shut off the blue light and work elsewhere.
- Mark small lesions early during procedure.
Uncertainty
around a large
pink/red area
around a large
pink/red area
- Direct scope 90° toward lesion. Fill bladder slightly. Stretch area with loop and see if it disappears.
Questions: - Has a cytology been taken? Were bacterial cultures taken? Has patient used catheter for longer periods?
- Is carcinoma in situ (CIS) suspected? Cold cup biopsy and no fulguration
- Did the patient have a positive urine cytology? Treat as CIS.
Resection management
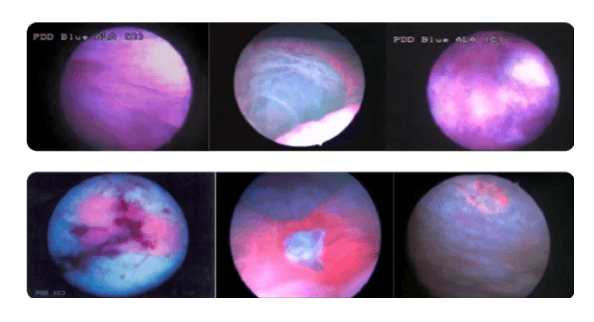
To avoid false-negative diagnoses
- Ensure adequate time after instillation of Cysview.
- Pink fluorescence on bladder neck should always be seen if the instillation was administered properly
Bladder neck
Fluorescence is considered normal and not necessarily tumor.
For more information, please access the program below.
Shining a light on Blue Light Cystoscopy with Cysview®:
What you need to know
Access this exclusive educational program focusing on the pros and cons of BLC of Cysview for the evaluation, diagnosis, and management of patients with
non-muscle invasive bladder cancer (NMBIC).
This program comprises two modules.
Module One:
Blue light vs. White Light Cystoscopy in the Management of Bladder Cancer — An expert roundtable discussion in which our expert panel share their views and experience and present thought-provoking case studies.
Roundtable participants:
- Badrinath Konety, MD, MBA [chair/moderator]
- Sia Daneshmand, MD
- Per-Uno Malmström, MD, PhD
Module Two:
NMIBC: A Patient’s Journey. Introduction to Blue Light Cystoscopy with Cysview with Tips and Tricks — An eSeries presentation featuring a patient case. This multi-modular program offers real-world tips and tricks from experts, delivers comprehensive discussions concerning management of NMIBC, and imparts valuable knowledge, particularly how Cysview integrates into the evaluation, diagnosis, surveillance and follow-up of patients with NMIBC.
Want to learn more?
References
1. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021–1029. Amended 2020. 2. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer. Version 3.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed June 28, 2023. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 3. Cysview [prescribing information]. 2019:1–4. 4. Witjes JA, Babjuk M, Gontero P, et al. Clinical and Cost Effectiveness of Hexaminolevulinate-Guided Blue-Light Cystoscopy: Evidence Review and Updated Expert Recommendation. Eur Urol. 2014;66(5):863–871. 5. Daneshmand S, Schuckman AK, Bochner BH, et al. Hexaminolevulinate Blue-Light Cystoscopy in Non-Muscle Invasive Bladder Cancer: Review of the Clinical Evidence and Consensus Statement on Appropriate Use in the USA. Nat Rev Urol. 2014;11(10):589–596 6. Lotan Y, Bivalacqua TJ, Downs T, et al. Blue Light Flexible Cystoscopy with Hexaminolevulinate in Non-Muscle Invasive Bladder Cancer: Review of the Clinical Evidence and Consensus Statement on Optimal Use in the USA—Update 2018. Nat Rev Urol. 2019;16(6):377–386. 7. Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate Guided Fluorescence Cystoscopy Reduces Recurrence in Patients with Nonmuscle Invasive Bladder Cancer. J Urol. 2010;184(5):1907–1914. 8. Daneshmand S, Patel S, Lotan Y, et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate (HAL) in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multi-Center Study. J Urol. 2018;199(5):1158–1165.
Anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis, and abnormal urinalysis have been reported after administration of Cysview. The most common adverse reactions seen in clinical trials were bladder spasm, dysuria, hematuria, and bladder pain.
Product indication for Cysview® (hexaminolevulinate HCl)
Cysview is an optical imaging agent indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder.
Cysview is used with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system to perform Blue Light Cystoscopy (BLC®) as an adjunct to White Light Cystoscopy.
Important Risk & Safety Information
Limitations of use
Cysview is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer.
Warnings and precautions
Anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis, and abnormal urinalysis have been reported after administration of Cysview. The most common adverse reactions seen in clinical trials were bladder spasm, dysuria, hematuria, and bladder pain.
Contraindications
Cysview should not be used in patients with porphyria, gross hematuria, or with known hypersensitivity to hexaminolevulinate or any derivative of aminolevulinic acid. Cysview may fail to detect some malignant lesions. False-positive fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, and recent BCG therapy or intravesical chemotherapy. No specific drug interaction studies have been performed.
Use in specific populations
Safety and effectiveness have not been established in pediatric patients. There are no available data on Cysview use in pregnant women. Adequate reproductive and developmental toxicity studies in animals have not been performed. Systemic absorption following administration of Cysview is expected to be minimal. There are no data on the presence of hexaminolevulinate in human or animal milk, the effects on a breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Cysview and any potential adverse effects on the breastfed infant from Cysview or from the underlying maternal condition.
Use of the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system
Cysview is approved for use with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system. For system set up and general information for the safe use of the PDD system, please refer to the KARL STORZ instruction manuals for each of the components.
Prior to Cysview administration, read the Full Prescribing Information and follow the preparation and reconstitution instructions.
BLC® and Cysview® are registered
trademarks of Photocure ASA
©2025 Photocure/Cysview. All Rights Reserved.
The information on this website is intended for U.S audiences only.
Residents of Canada, please visit Cysview.ca.

