Gain the edge
against bladder cancer
with Blue Light Cystoscopy
with Cysview®
Cysview® is clinically proven to detect
non-muscle invasive bladder cancer
(NMIBC) missed by white light alone.
Cysview® is clinically proven to detect
non-muscle invasive bladder cancer
(NMIBC) missed by white light alone.
About Cysview®
What is it?
Cysview is an FDA-approved product that makes bladder cancer tumors glow bright pink under blue light so urologists can remove them more completely than if they weren’t using Cysview.
Who is it for?
Cysview is for use in patients with NMIBC. It should be used for the first TURBT and for all intermediate and high-risk NMIBC patients during surgical treatment and surveillance/follow-up.
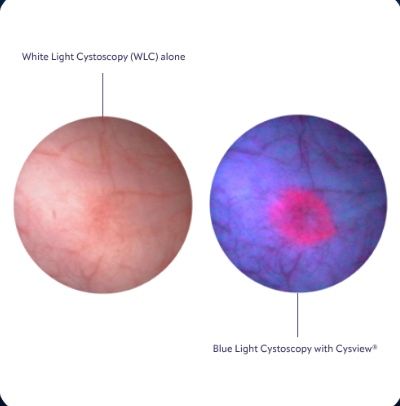
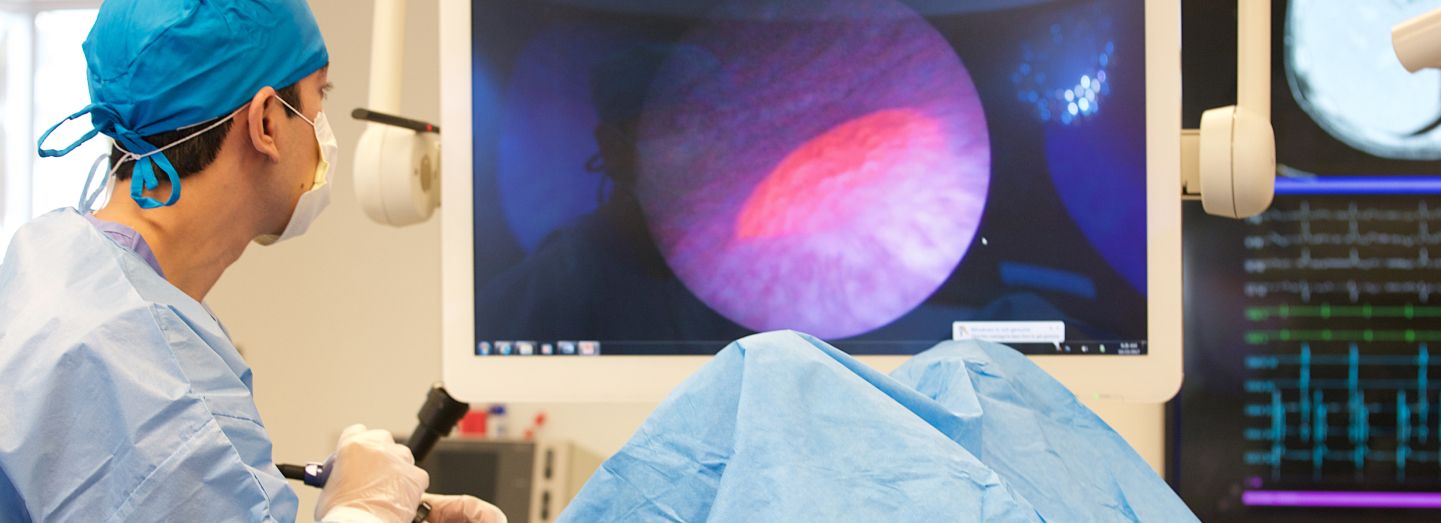
See the difference
With Cysview, NMIBC tumors are much more visible during a cystoscopy. In blue light, Cysview makes them glow bright pink.
BLC® with Cysview® offers many benefits
- You will be more confident in your detection and management of NMIBC in intermediate and high-risk patients.
- Your patients will be more confident in their care.
- You can be confident in this state-of-the-art product and the support provided for using it in your practice.

What is Cysview®?
Cysview is a pharmaceutical product that makes non-muscle invasive bladder cancer (NMIBC) tumors glow bright pink under blue light during a cystoscopy.1
It was approved by the US FDA in June 2010.
The Blue Light Cystoscopy (BLC®) procedure uses both white and blue light for enhanced visibility of NMIBC tumors.1

Indication
Cysview is an optical imaging agent indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder.
Cysview is used with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system to perform Blue Light Cystoscopy (BLC®) as an adjunct to White Light Cystoscopy (WLC).
Why is there a need for Cysview®?




Why is there a need for Cysview®?
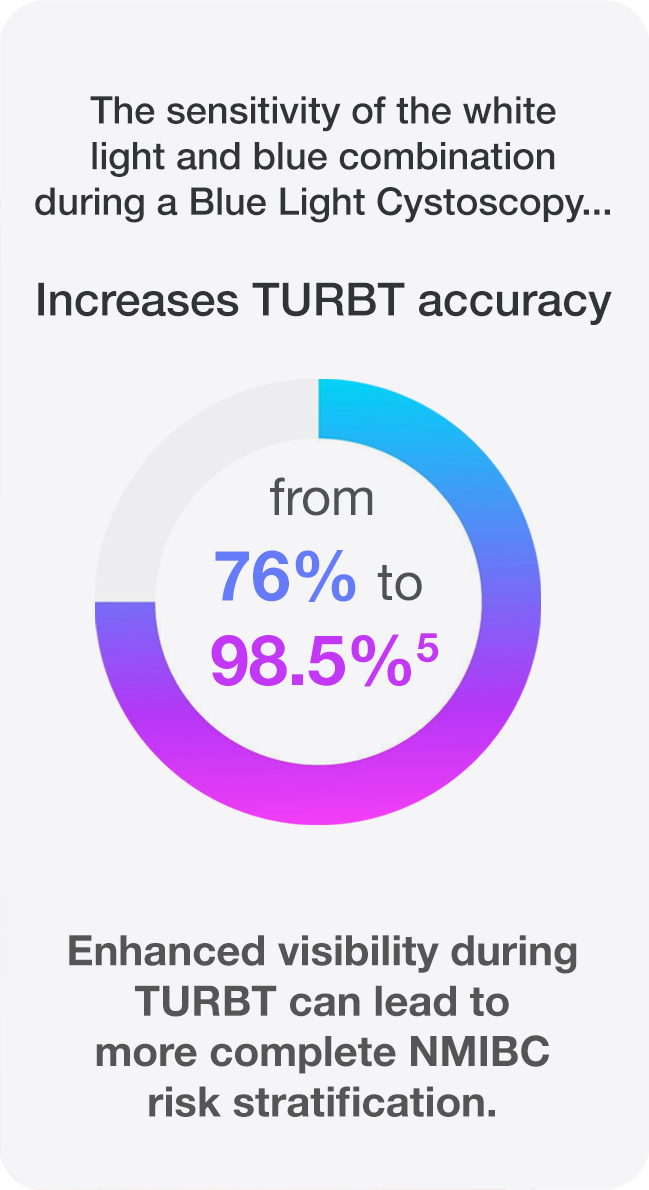
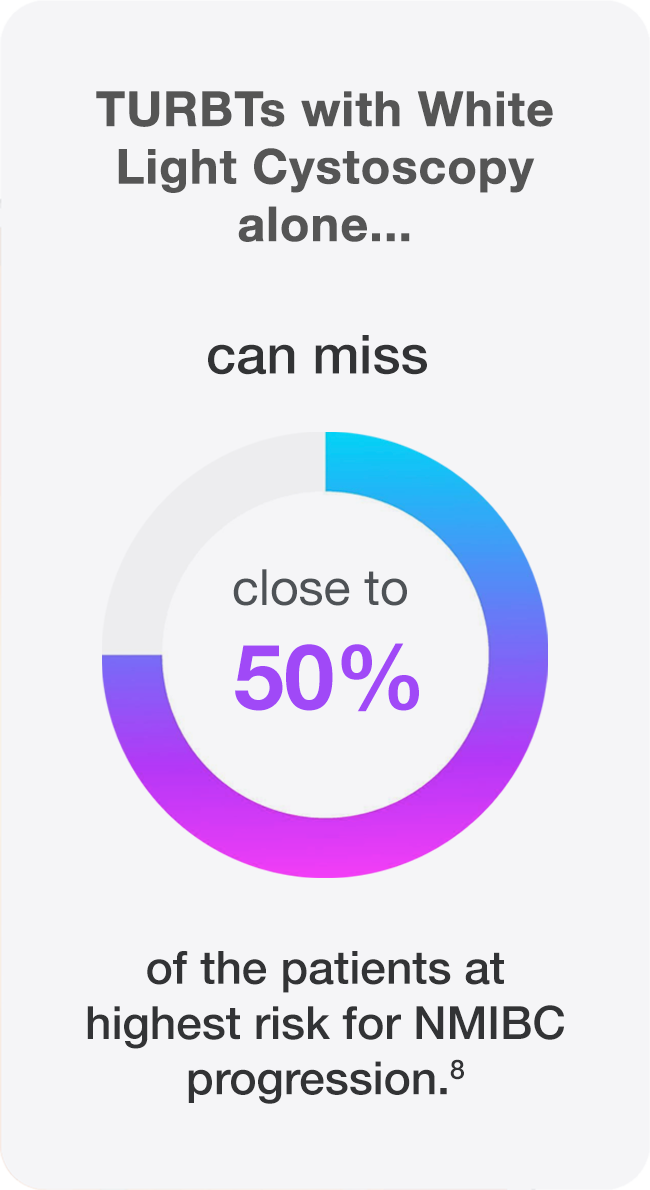
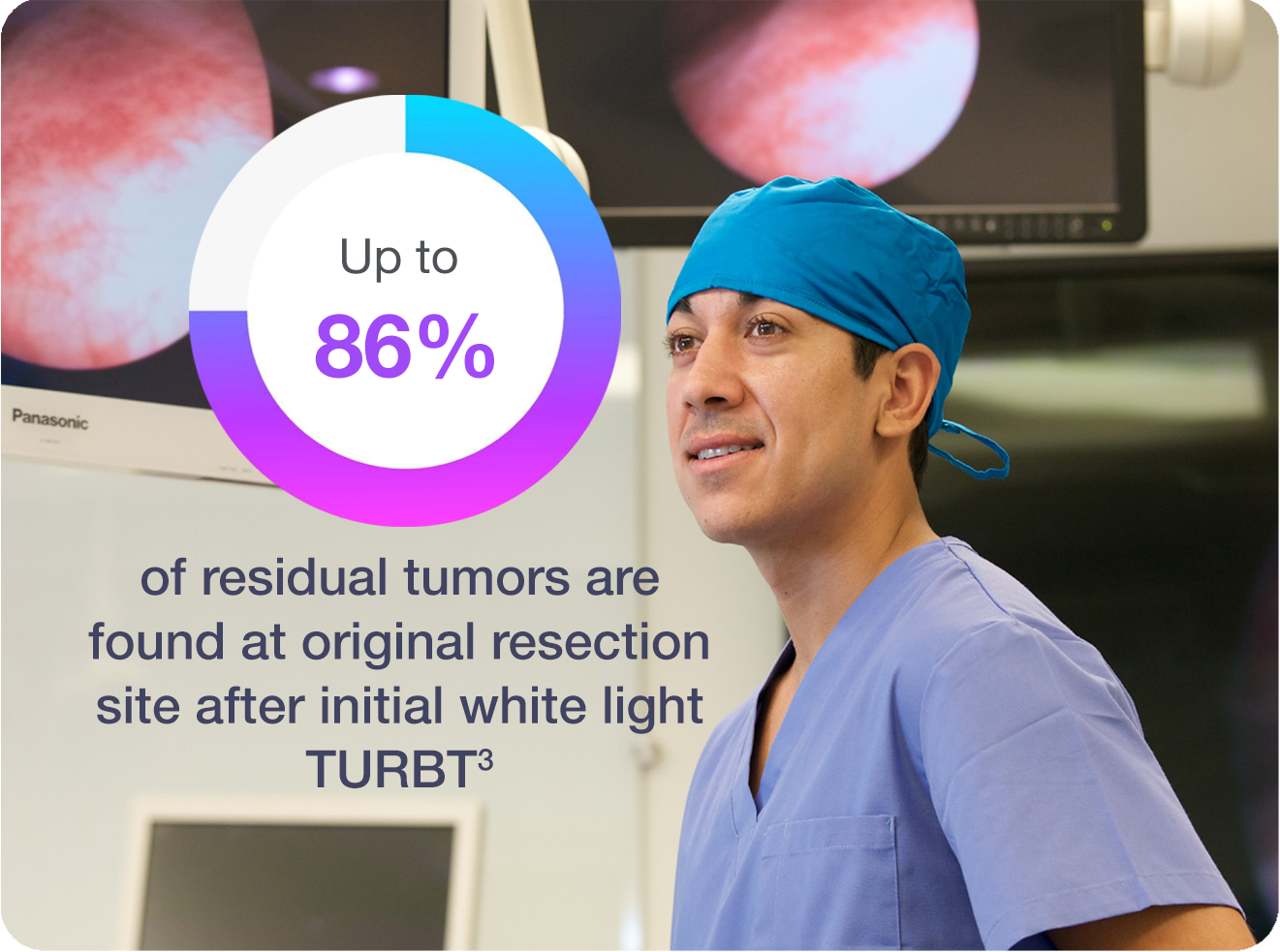
The standard technique for detection of bladder cancer, (WLC), can miss tumors that are there but not visible.2 When TURBT is performed with only white light, up to 75% of patients present with tumors at repeat TURBT.3
When TURBT is
performed with only
white light
present with tumors
at repeat TURBT3
Why is there a need for Cysview®?










How can Cysview® help?
BLC® with Cysview:
- Helps enhance tumor margins, increasing confidence in complete resection.4,5
- Is the most studied enhanced cystoscopy with the only mechanism of action that targets cancer cells.1,6
BLC with Cysview is FDA-approved for use in both TURBT and
surveillance1 and is approved for use with a:
Rigid cystoscope
(operating room/TURBT procedure/follow-up)
Flexible cystoscope (office/surveillance/follow-up)
Cysview® during TURBT
BLC with Cysview offers optimal diagnosis right from the first TURBT:
- Detects more tumors than white light alone.1
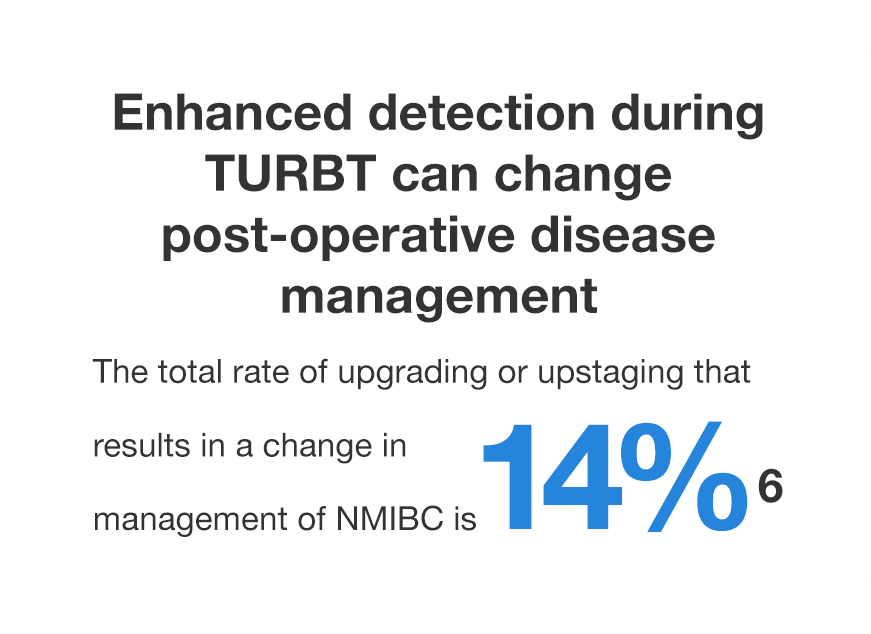
- Better detection may lead to appropriate risk categorization.7
Cysview may not detect all malignant lesions.
Cysview® in a surveillance setting
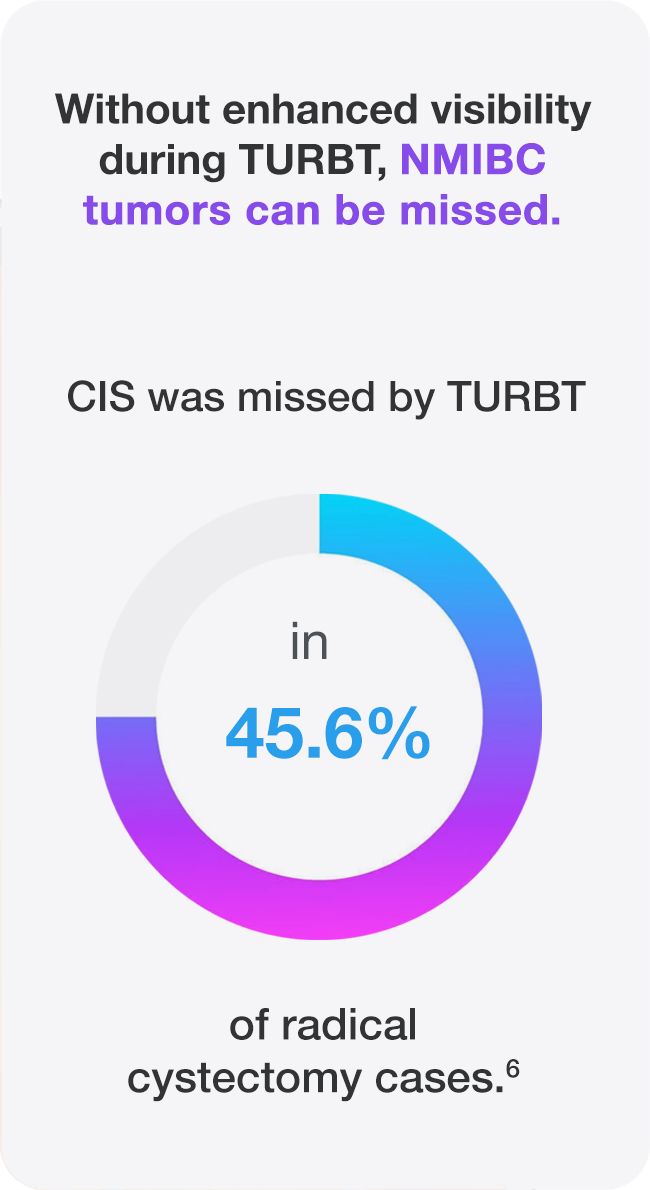
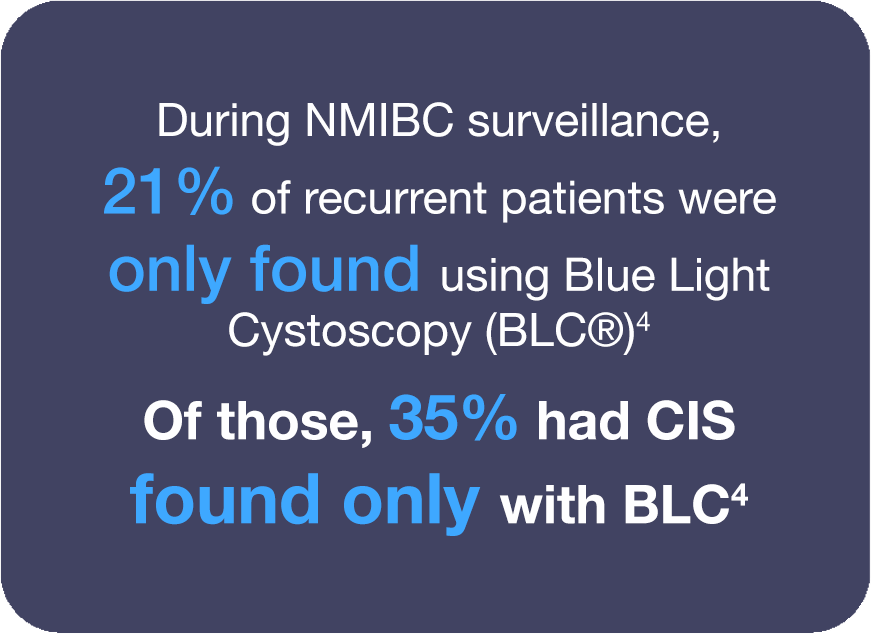
In the phase III study that resulted in the expanded Cysview indication to include patients undergoing surveillance cystoscopy for carcinoma of the bladder, results showed that Cysview significantly improves detection of patients with recurrent bladder cancer (p<0.0001).1 Experts recommend using BLC with Cysview for surveillance.7
Well-established safety profile
with over a decade of data1
The most common adverse reactions reported in patients who received Cysview (≤2% of patients):
- Bladder spasm
- Hematuria
- Procedural pain
- Headache
- Dysuria
- Bladder pain
- Urinary infection
The following adverse reactions have been voluntarily reported during post-approval use of Cysview. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Anaphylactoid shock
- Bladder pain
- Abnormal urinalysis
- Hypersensitivity reactions
- Cystitis
No specific drug interaction studies have been performed.
Warnings and precautions:
- Anaphylaxis: have trained personnel and therapies available.
- Failed detection: Cysview may not detect all malignant lesions. Always perform WLC followed by BLC. Do not biopsy with blue light only.
- False fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, recent BCG therapy or chemotherapy.
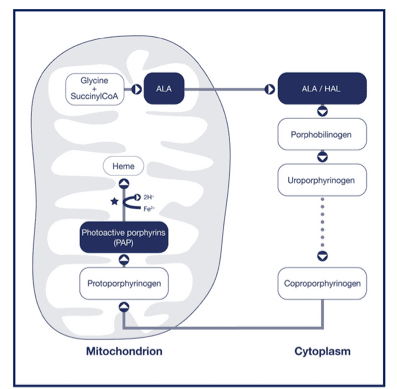
How Cysview® works
Mechanism of action pathway8
- After instillation in the bladder, Cysview interacts with the heme biosynthetic pathway.
- Selective intracellular accumulation of photoactive porphyrins (PAP) takes place.
- PAPs preferentially accumulate in rapidly proliferating neoplastic cells.
- After one hour, sufficient PAPs accumulate.
- Under subsequent blue light illumination, neoplastic cells fluoresce bright pink.
See the difference
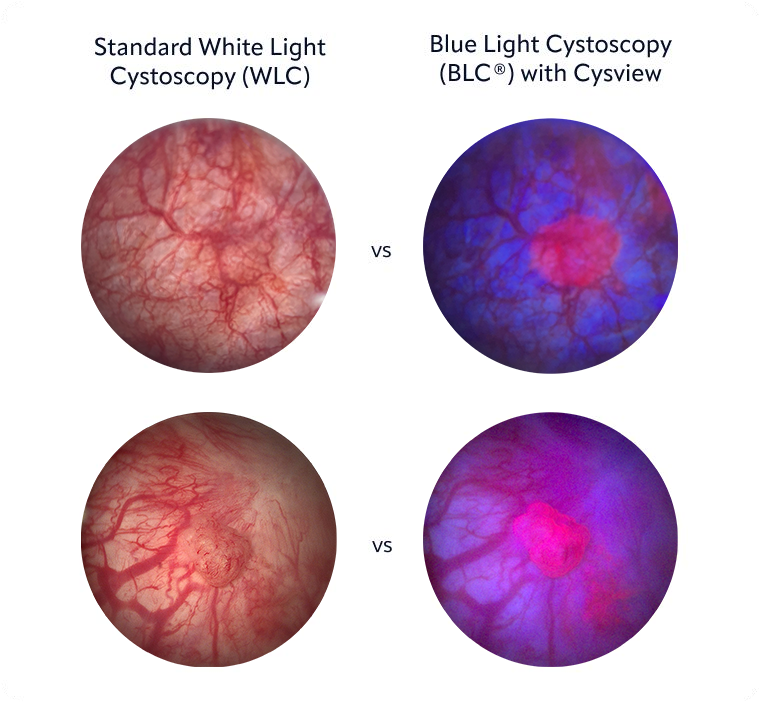
Cysview may not detect all malignant lesions. False-positive fluorescence may occur due to
inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, recent BCG immunotherapy or
intravesical chemotherapy.
References
1. Cysview [prescribing information]. 2019:1–4. 2. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021–1029. Amended 2020. 3. Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat Transurethral Resection in Non-Muscle Invasive Bladder Cancer: A Systematic Review. Eur Urol. 2018;73(6):925–933. 4. Daneshmand S, Patel S, Lotan Y, et al. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. J Urol. 2018;199(5):1158-1165. 5. Witjes JA, Babjuk M, Gontero P, et al. Clinical and Cost Effectiveness of Hexaminolevulinate-Guided Blue-Light Cystoscopy: Evidence Review and Updated Expert Recommendations. Eur Urol. 2014;66(5):863-871. 6. Xiong Y, Li J, Ma S, et al. A Meta-analysis of Narrow Band Imaging for the Diagnosis and Therapeutic Outcome of Non-Muscle Invasive Bladder Cancer. PLoS One. 2017;12(2):e0170819. 7. Richards KA, Smith ND, Steinberg GD. The Importance of Transurethral Resection of Bladder Tumor in the Management of Nonmuscle Invasive Bladder Cancer: A Systematic Review of Novel Technologies. J Urol. 2014;191(6):1655-1664. 8. Wachoska M, Muchowicz A, Firczuk M, et al. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules. 2011;16(5):4140–4164.
Anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis, and abnormal urinalysis have been reported after administration of Cysview. The most common adverse reactions seen in clinical trials were bladder spasm, dysuria, hematuria, and bladder pain.
Product indication for Cysview® (hexaminolevulinate HCl)
Cysview is an optical imaging agent indicated for use in the cystoscopic detection of carcinoma of the bladder, including carcinoma in situ (CIS), among patients suspected or known to have lesion(s) on the basis of a prior cystoscopy, or in patients undergoing surveillance cystoscopy for carcinoma of the bladder.
Cysview is used with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system to perform Blue Light Cystoscopy (BLC®) as an adjunct to White Light Cystoscopy.
Important Risk & Safety Information
Limitations of use
Cysview is not a replacement for random bladder biopsies or other procedures used in the detection of bladder cancer.
Warnings and precautions
Anaphylactoid shock, hypersensitivity reactions, bladder pain, cystitis, and abnormal urinalysis have been reported after administration of Cysview. The most common adverse reactions seen in clinical trials were bladder spasm, dysuria, hematuria, and bladder pain.
Contraindications
Cysview should not be used in patients with porphyria, gross hematuria, or with known hypersensitivity to hexaminolevulinate or any derivative of aminolevulinic acid. Cysview may fail to detect some malignant lesions. False-positive fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue, previous bladder biopsy, and recent BCG therapy or intravesical chemotherapy. No specific drug interaction studies have been performed.
Use in specific populations
Safety and effectiveness have not been established in pediatric patients. There are no available data on Cysview use in pregnant women. Adequate reproductive and developmental toxicity studies in animals have not been performed. Systemic absorption following administration of Cysview is expected to be minimal. There are no data on the presence of hexaminolevulinate in human or animal milk, the effects on a breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Cysview and any potential adverse effects on the breastfed infant from Cysview or from the underlying maternal condition.
Use of the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system
Cysview is approved for use with the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system. For system set up and general information for the safe use of the PDD system, please refer to the KARL STORZ instruction manuals for each of the components.
Prior to Cysview administration, read the Full Prescribing Information and follow the preparation and reconstitution instructions.
BLC® and Cysview® are registered
trademarks of Photocure ASA
©2025 Photocure/Cysview. All Rights Reserved.
The information on this website is intended for U.S audiences only.
Residents of Canada, please visit Cysview.ca.

