Ordering
How to order Cysview®
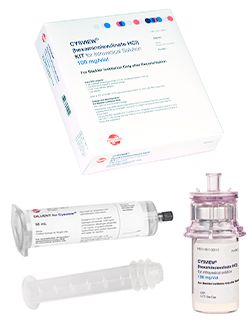
Cysview is packaged in a kit that includes:
- One 100 mg vial of Cysview powder.
- One vial adapter.
- One prefilled syringe containing 50 mL diluent for Cysview.
- One syringe plunger.
- One Luer Lock catheter adapter.
For current customers
We definitely appreciate your business.
To order more Cysview, please email or call toll-free using the information below.
Cysview Customer Service
- 1-855-CYSVIEW (1-855-297-8439) | 8 AM–7 PM CST Monday–Friday
Choose option 1 for new orders or to inquire about an existing order or invoice
You will need:
- Description: CYSVIEW 100mg kit.
- Product code: 3001-2.
- NDC#: 10511-3001-2.
For ordering ease, you should have the following:
- Account name.
- Contact name (person who is placing the order).
- Contact phone number.
- Ship to address.
- Purchase order number.
- Photocure account number, if available.
Choose option 2 for other topics
- Medical affairs.
- Reporting an adverse event.
- Lodging a product complaint.
- Requesting clinical information.
- Any product-related requests.
For any additional needs, please contact your Cysview Account Manager. Thank you.
Terms and conditions
Minimum order quantity is 9 kits, unless lower quantity is approved by Photocure Operations Director. Payment terms are 30 days, unless exception is approved by Photocure Finance Director. No returned goods accepted, nor credit memo issued, except for product shipped in error, product damaged in transit or a product recall. Products shipped in error by Photocure, Inc. or products damaged in transit must be reported to Photocure, Inc. within ten (10) working days of receipt and must be returned to Photocure, Inc. within 25 days of receipt. Contact Photocure, Inc. customer service at 855-297-8439 phone or 414-434-6952 fax. Shipping FOB destination by ground standard delivery with prepaid freight passed through to customer. All orders received by 2 pm CST will ship next business day. Customer agrees to pay expediting fee and overnight shipping fee if those services are requested. Late payment interest 1% per month. Check or ACH accepted by contacting Photocure Accounting Manager at 609-627-9309.
EVERSANA holiday schedule - closures 2024
- Jan 01 New Year's Day
- Jan 15 Martin Luther King Jr. Day
- May 27 Memorial Day
- June 19 Juneteenth Day
- July 04 Independence Day
- Sept 02 Labor Day
- Nov 28 Thanksgiving Day
- Nov 29 Thanksgiving Friday
- Dec 24 Christmas Eve
- Dec 25 Christmas Day

